## Why Is Nitrogen in the Atmosphere Not Used by Plants and Animals? A Short Response & In-Depth Explanation
You’ve likely heard that nitrogen is essential for life. Indeed, it’s a crucial component of proteins, DNA, and other vital biomolecules. Given that the atmosphere is about 78% nitrogen gas (N₂), it’s natural to wonder: *why is nitrogen in the atmosphere not used by plants and animals? short response*? The simple answer is that atmospheric nitrogen is in a form that most organisms cannot directly utilize. This article delves into the reasons behind this paradox, exploring the complex processes that make nitrogen available to living organisms, and highlighting the critical role of nitrogen fixation in the global ecosystem. We will explore the science behind this, providing you with a complete understanding of nitrogen’s role and its limitations.
Unlike some other elements, nitrogen in its diatomic form (N₂) is exceptionally stable due to the strong triple bond between the two nitrogen atoms. This stability makes it inert, meaning it doesn’t readily react with other substances. While this inertness is beneficial in many ways (preventing uncontrolled reactions in the atmosphere, for example), it also prevents plants and animals from directly incorporating nitrogen into their tissues. The process of breaking this strong triple bond and converting N₂ into a usable form is called nitrogen fixation, and it’s a crucial step in the nitrogen cycle.
This comprehensive guide will provide a detailed explanation of the nitrogen cycle, the organisms responsible for nitrogen fixation, and the various pathways that nitrogen takes as it moves through the environment. We’ll also address common misconceptions about nitrogen use and explore the implications of nitrogen pollution. By the end of this article, you’ll have a thorough understanding of why atmospheric nitrogen is not directly available to most life forms and the fascinating processes that make it accessible.
### Deep Dive: Understanding Nitrogen’s Unavailability
The atmosphere is a vast reservoir of nitrogen, but this nitrogen exists primarily as dinitrogen (N₂), a molecule composed of two nitrogen atoms held together by a strong triple covalent bond. This bond is one of the strongest in nature, requiring a significant amount of energy to break. This is the core reason *why is nitrogen in the atmosphere not used by plants and animals? short response*. Plants and animals lack the enzymatic machinery to break this bond, rendering atmospheric nitrogen inaccessible to them.
To understand this better, let’s consider the chemical properties of nitrogen. Nitrogen has five valence electrons, allowing it to form up to three covalent bonds. In dinitrogen, each nitrogen atom shares three electrons with the other, resulting in a triple bond (N≡N). This triple bond has a bond energy of approximately 945 kJ/mol, which is significantly higher than the bond energies of single or double bonds. This high bond energy means that it requires a substantial input of energy to cleave the bond and make nitrogen atoms available for other chemical reactions.
**Core Concepts & Advanced Principles**
* **Bond Energy & Reactivity:** The high bond energy of dinitrogen directly correlates with its low reactivity. Molecules with high bond energies are generally stable and unreactive, while those with lower bond energies are more prone to chemical reactions.
* **Enzymatic Limitations:** Plants and animals rely on enzymes to catalyze biochemical reactions. However, they lack the specific enzymes required to break the triple bond of dinitrogen. These enzymes are highly specialized and only found in certain microorganisms.
* **Thermodynamic Constraints:** Even if plants and animals possessed the necessary enzymes, the direct assimilation of dinitrogen would be thermodynamically unfavorable under normal conditions. The conversion of dinitrogen to ammonia (NH₃), a more usable form of nitrogen, requires a significant input of energy.
**Importance & Current Relevance**
The inability of plants and animals to directly use atmospheric nitrogen has profound ecological implications. It creates a fundamental dependency on nitrogen-fixing microorganisms, which act as the primary gatekeepers of nitrogen in the biosphere. Without these microorganisms, life as we know it would not be possible.
Furthermore, the nitrogen cycle is intricately linked to various environmental issues, including climate change, water pollution, and biodiversity loss. Human activities, such as the excessive use of nitrogen fertilizers, have disrupted the natural nitrogen cycle, leading to a cascade of negative consequences. Understanding why plants and animals cannot directly use atmospheric nitrogen is crucial for addressing these environmental challenges and developing sustainable agricultural practices.
### The Role of Nitrogen Fixation
Nitrogen fixation is the process by which atmospheric nitrogen (N₂) is converted into ammonia (NH₃), a form of nitrogen that can be used by plants and other organisms. This process is primarily carried out by certain bacteria and archaea, collectively known as diazotrophs. These microorganisms possess a unique enzyme called nitrogenase, which catalyzes the reduction of dinitrogen to ammonia. Nitrogen fixation is essential because it converts atmospheric nitrogen into a bioavailable form, making it accessible to plants and, indirectly, to animals.
**Nitrogenase: The Key Enzyme**
Nitrogenase is a complex enzyme that consists of two main components: the dinitrogenase reductase (Fe protein) and the dinitrogenase (MoFe protein). The Fe protein transfers electrons to the MoFe protein, which then reduces dinitrogen to ammonia. The nitrogenase enzyme is highly sensitive to oxygen and requires an anaerobic environment to function effectively. This is why nitrogen fixation often occurs in specialized structures or environments that minimize oxygen exposure.
**Types of Nitrogen Fixation**
* **Biological Nitrogen Fixation:** This is the most significant form of nitrogen fixation and is carried out by diazotrophs. These microorganisms can be free-living or symbiotic. Free-living diazotrophs, such as *Azotobacter* and *Clostridium*, fix nitrogen independently in the soil. Symbiotic diazotrophs, such as *Rhizobium*, form a mutually beneficial relationship with plants, typically legumes, in specialized structures called root nodules.
* **Industrial Nitrogen Fixation:** This process, also known as the Haber-Bosch process, converts atmospheric nitrogen and hydrogen into ammonia using high pressure and temperature in the presence of a catalyst. The Haber-Bosch process has revolutionized agriculture by providing a readily available source of nitrogen fertilizer, but it also has significant environmental impacts due to its high energy consumption and contribution to nitrogen pollution.
* **Atmospheric Nitrogen Fixation:** Lightning strikes can also fix atmospheric nitrogen by providing the energy needed to break the triple bond of dinitrogen. The resulting nitrogen oxides can then react with water to form nitrates, which are deposited in the soil through rainfall.
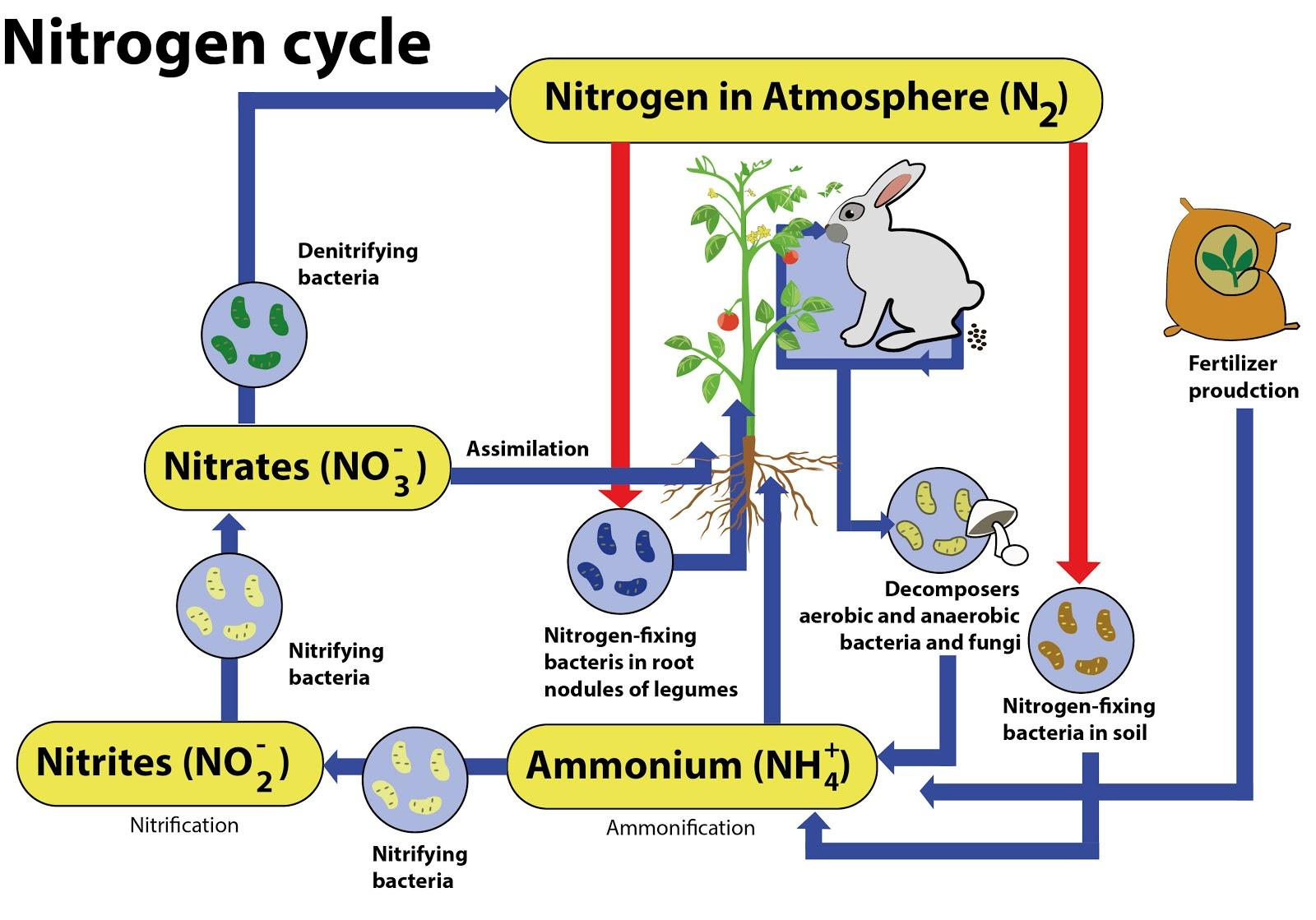
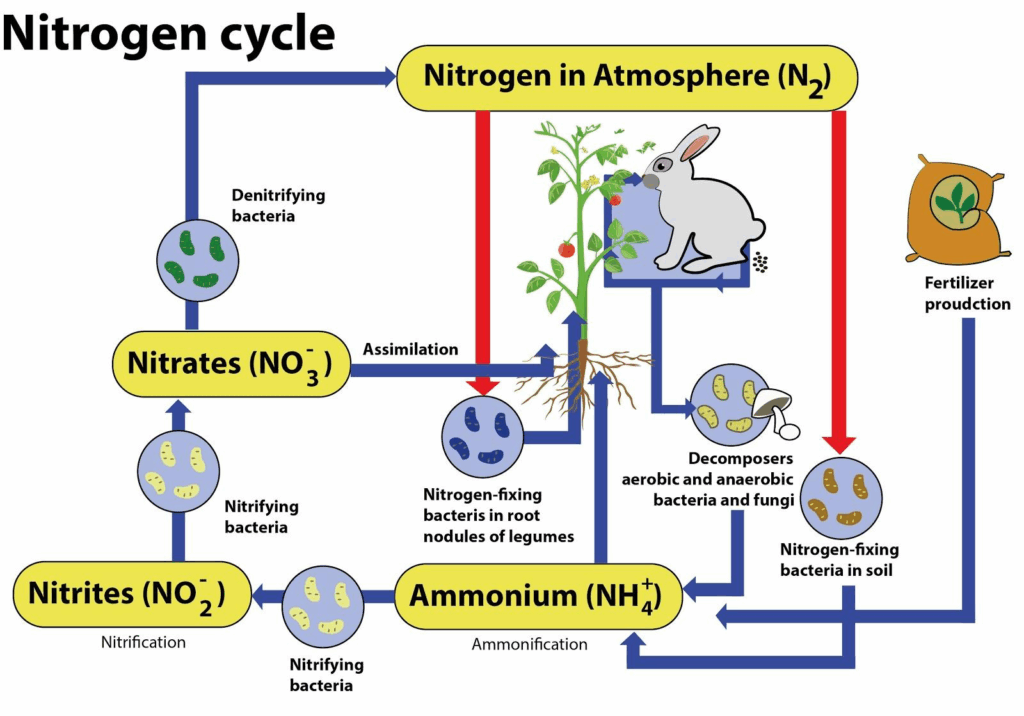
### The Nitrogen Cycle: A Detailed Overview
The nitrogen cycle is a complex biogeochemical cycle that describes the transformations of nitrogen in the environment. It involves several key processes, including nitrogen fixation, ammonification, nitrification, and denitrification. Understanding the nitrogen cycle is essential for comprehending why atmospheric nitrogen is not directly used by plants and animals and how nitrogen moves through the ecosystem.
**Key Processes in the Nitrogen Cycle**
* **Nitrogen Fixation:** As previously discussed, this process converts atmospheric nitrogen into ammonia, making it available to plants and other organisms.
* **Ammonification:** This is the process by which organic nitrogen (e.g., from dead plants and animals) is converted into ammonia. Ammonification is carried out by decomposers, such as bacteria and fungi, which break down organic matter and release ammonia into the soil.
* **Nitrification:** This is a two-step process by which ammonia is converted into nitrate (NO₃⁻), a form of nitrogen that is readily taken up by plants. Nitrification is carried out by nitrifying bacteria, which oxidize ammonia to nitrite (NO₂⁻) and then nitrite to nitrate.
* **Denitrification:** This is the process by which nitrate is converted back into atmospheric nitrogen. Denitrification is carried out by denitrifying bacteria, which thrive in anaerobic conditions. Denitrification plays a crucial role in removing excess nitrogen from the environment and preventing nitrogen pollution.
* **Assimilation:** This is the process by which plants and other organisms incorporate ammonia or nitrate into their tissues. Plants use nitrogen to synthesize proteins, nucleic acids, and other essential biomolecules. Animals obtain nitrogen by consuming plants or other animals.
### Consequences of Disrupting the Nitrogen Cycle
The nitrogen cycle is a delicate balance, and human activities have significantly disrupted this balance, leading to a range of environmental problems. The excessive use of nitrogen fertilizers, the burning of fossil fuels, and deforestation have all contributed to increased nitrogen pollution.
**Environmental Impacts of Nitrogen Pollution**
* **Eutrophication:** Excess nitrogen in aquatic ecosystems can lead to eutrophication, which is the excessive growth of algae and other aquatic plants. This can deplete oxygen levels in the water, leading to the death of fish and other aquatic organisms.
* **Acid Rain:** Nitrogen oxides released from burning fossil fuels can react with water in the atmosphere to form nitric acid, which contributes to acid rain. Acid rain can damage forests, acidify lakes and streams, and corrode buildings and monuments.
* **Greenhouse Gas Emissions:** Nitrous oxide (N₂O), a byproduct of denitrification, is a potent greenhouse gas that contributes to climate change. Agricultural activities are a major source of nitrous oxide emissions.
* **Air Pollution:** Nitrogen oxides are also major air pollutants that contribute to smog and respiratory problems.
### Q&A: Addressing Your Nitrogen-Related Questions
Here are some common questions about nitrogen and its role in the environment:
1. **Why can’t plants directly absorb nitrogen gas from the air through their leaves?** Plants lack the necessary enzymes to break the strong triple bond in N₂ gas. They can only absorb nitrogen in the form of ammonium (NH₄⁺) or nitrate (NO₃⁻) through their roots.
2. **Are there any plants that can directly use atmospheric nitrogen?** No, all plants rely on nitrogen-fixing microorganisms to convert atmospheric nitrogen into a usable form. However, some plants, such as legumes, have a symbiotic relationship with nitrogen-fixing bacteria in their root nodules.
3. **What is the role of lightning in the nitrogen cycle?** Lightning can fix atmospheric nitrogen by providing the energy needed to break the triple bond of dinitrogen. The resulting nitrogen oxides can then react with water to form nitrates, which are deposited in the soil through rainfall.
4. **How does the Haber-Bosch process impact the nitrogen cycle?** The Haber-Bosch process has significantly altered the nitrogen cycle by providing a readily available source of nitrogen fertilizer. While this has increased agricultural productivity, it has also led to increased nitrogen pollution and environmental problems.
5. **What are some sustainable agricultural practices that can reduce nitrogen pollution?** Sustainable agricultural practices include using cover crops, crop rotation, reduced tillage, and precision fertilization. These practices can help to reduce nitrogen losses from agricultural fields and minimize nitrogen pollution.
6. **How does deforestation affect the nitrogen cycle?** Deforestation can disrupt the nitrogen cycle by reducing the amount of organic matter in the soil and increasing the rate of nitrogen loss through leaching and runoff. Deforestation can also lead to increased emissions of nitrous oxide, a potent greenhouse gas.
7. **What is the difference between nitrification and denitrification?** Nitrification is the process by which ammonia is converted into nitrate, while denitrification is the process by which nitrate is converted back into atmospheric nitrogen. Nitrification is carried out by nitrifying bacteria, while denitrification is carried out by denitrifying bacteria.
8. **How does nitrogen pollution affect human health?** Nitrogen pollution can affect human health through several pathways, including drinking water contamination, air pollution, and the consumption of food contaminated with nitrates. High levels of nitrates in drinking water can cause methemoglobinemia, a condition that reduces the ability of blood to carry oxygen.
9. **What are some ways to reduce nitrogen emissions from vehicles?** Reducing nitrogen emissions from vehicles can be achieved through the use of catalytic converters, improved engine design, and the use of alternative fuels. Regular vehicle maintenance and proper tire inflation can also help to reduce nitrogen emissions.
10. **What role do wetlands play in the nitrogen cycle?** Wetlands play an important role in the nitrogen cycle by acting as natural filters that remove excess nitrogen from the water. Wetlands can support high rates of denitrification, which helps to convert nitrate back into atmospheric nitrogen.
### Conclusion: Embracing the Nitrogen Cycle’s Complexity
In summary, *why is nitrogen in the atmosphere not used by plants and animals? short response* boils down to the strong triple bond in dinitrogen (N₂) and the lack of necessary enzymes in plants and animals to break this bond. Nitrogen fixation, primarily carried out by microorganisms, is the crucial process that converts atmospheric nitrogen into a usable form. Understanding the nitrogen cycle and the impacts of human activities on this cycle is essential for developing sustainable practices that protect our environment.
By grasping the intricacies of nitrogen’s role in the biosphere, we can better appreciate the delicate balance of our ecosystems and work towards mitigating the negative consequences of nitrogen pollution. Share your thoughts and experiences with nitrogen management in the comments below. Further explore our resources to understand the broader implications on sustainable agriculture.